-
Inspiracje
- Najnowsze
- Najpopularniejsze
- Zaskocz mnie
- Obserwowane
- MAGazyn
- Moda damska
- DIY - zrób to sam
- Kuchnia
- Uroda
- Wnętrza
- Humor
- Ogród
- Ślub
- Architektura
- Biżuteria
- Design
- Dziecko
- Film
- Fotografia
- Gadżety
- Historia
- Hobby
- Inne
- Książka
- Moda męska
- Muzyka
- Nauka i natura
- Plakaty i obrazy
- Podróże i miejsca
- Samochody i motocykle
- Sport i fitness
- Święta i uroczystości
- Tatuaż
- Zwierzęta
- Partnerzy
- KONKURS
-
Zakupy
-
ONA
- Ubrania
- Bielizna
- Bluzki
- Bluzy
- Dresy damskie
- Kombinezony
- Koszule
- Koszulki
- Kurtki
- Płaszcze
- Spodnie i leginsy
- Spodnie jeans
- Spódnice
- Stroje kąpielowe
- Sukienki i tuniki
- Swetry
- Szorty
- Zestawy
- Żakiety i kamizelki
- Buty
- Baleriny
- Botki
- Czółenka
- Espadryle
- Glany
- Japonki
- Kalosze
- Klapki
- Koturny
- Kowbojki
- Kozaki
- Obuwie Domowe
- Półbuty
- Sandały
- Sportowe i lifestyle
- Szpilki
- Tenisówki i Trampki
- Trapery i trekkingi
- Dodatki
- Bransoletki i zawieszki
- Breloki
- Czapki i kapelusze
- Etui
- Kolczyki, nausznice
- Kołnierzyki
- Kosmetyczki
- Naszyjniki, kolie i zawieszki
- Okulary
- Ozdoby do włosów
- Parasole
- Paski
- Pierścionki
- Plecaki
- Portfele
- Rękawiczki
- Skarpety
- Szaliki i chusty
- Torby i torebki
- Walizki
- Zegarki
- Zestawy biżuterii
- Zdrowie i uroda
- Balsamy
- Bazy i podkłady
- Cienie do oczu
- Dezodoranty
- Higiena jamy ustnej
- Korektory
- Kredki
- Kremy i serum
- Lakiery
- Manicure i pedicure
- Maseczki
- Odżywki
- Peeling
- Perfumy i wody perfumowane
- Pielęgnacja po opalaniu
- Płyny żele i mydła
- Pomadki i błyszczyki
- Pudry
- Róże
- Stylizacja włosów
- Szampony
- Toniki i demakijaż
- Tusze
- Wody toaletowe
- Zestawy
-
ON
- Ubrania
- Bielizna
- Bluzy
- Kąpielówki
- Koszule
- Kurtki i płaszcze
- Marynarki kamizeki
- Spodenki i szorty
- Spodnie
- Swetry
- T-shirt długi rękaw
- T-shirt i Polo
- KULTURA
- GADŻETY
- DZIECKO
-
DOM I WNĘTRZE
- Wnętrza
- Akcesoria dom
- Ceramika i szkło
- Do baru
- Do biura
- Do kuchni
- Kubki kufle i kieliszki
- Lustra
- Nakrycia stołu
- Oświetlenie
- Plakaty i tablice
- Pościele, poduszki i nakrycia
- Przechowywanie
- Tekstylia
- Zegary i budziki
-
Marki
- MARKI
- Adidas
- Adriatica
- Alter Core
- Armani
- Asics
- Atlantic
- Azzaro
- Barbie
- Bburago
- Be-U
- Benetton
- Bering
- Black Plum
- Burberry
- Bvlgari
- Cacharel
- Calvin Klein
- Canon
- Carolina Herrera
- Carrera
- Casio
- Celestron
- Chanel
- Chloe
- Clinique
- Cobi
- Coloud
- Converse
- David Beckham
- Davidoff
- Delbana
- Diesel
- Dior
- DISNEY
- DKNY
- Dolce & Gabbana
- DOXA
- Dr. Martens
- Dunhill
- Ecco
- Elizabeth Arden
- Esprit
- EVC DSGN
- Festina
- Fila
- Fisher Price
- Frederique Constant
- Givenchy
- Gucci
- Guerlain
- Guess
- Hasbro
- Helena Rubinstein
- Hermes
- Hi-Tec
- Hugo Boss
- Hunter
- Iceberg
- Ingersoll
- Issey Miyake
- Jean Paul Gaultier
- Jennifer Lopez
- Jil Sander
- Jimmy Choo
- Joop!
- Juicy Couture
- Kappa
- Kenzo
- Komono
- Lacoste
- Lalique
- Lancome
- Lanvin
- Lee
- Lego
- Lorus
- Marc Jacobs
- Marshall
- Masaki Matsushima
- Matchbox
- Mattel
- Max Factor
- Max&Co
- Mc Arthur
- Mizuno
- Mont Blanc
- Moschino
- Mr. Gugu & Miss Go
- My Little Pony
- New Balance
- Nike
- Nikon
- Nina Ricci
- Ninety Eight Clothing
- Nivea
- Nixon
- Nooka
- Obaku
- Onitsuka Tiger
- Paco Rabanne
- Pentax
- Pewex
- Pierre Cardin
- Playboy
- Prada
- Puma
- Ralph Lauren
- Reebok
- Regatta
- Revlon
- Rexona
- Rider
- Salomon
- Salvatore Ferragamo
- Shelyak
- Shiseido
- Skagen
- Swarovski
- Takahashi
- Thierry Mugler
- Timberland
- Timex
- Tommy Hilfiger
- UGC
- Urbanears
- Valentino
- Vans
- Versace
- Viktor & Rolf
- Vixen
- Welly
- William Optics
- Wrangler
- Yimaida
- Yukon
- Yves Saint Laurent
-
ONA
-
Szukaj
- Losuj
Inne
protheragen
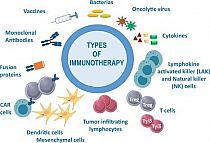
During the drug development and approval process, good clinical practice and regulatory compliance demand careful recording of clinical and non-clinical data in documents. Investigator's Brochure (IB) are complex documents that provide investigators and ethics committees with a broad overview of a product's development. All investigator brochures will be prepared in accordance with Appendix I (Investigator Brochure Format and Contents) which provides a model for the standard requirements. It is used by investigators to ensure protocol compliance and is often submitted to regulatory bodies as part of new drug applications. Investigator's Brochure Translation; IB Translation https://medtrans.protheragen.com/investigators-brochure-translation.html
MA==
Podobne inspiracje
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides comprehensive so…
Protheragen provides effective AI solutions for Image D…
Protheragen provides effective AI solutions for Surgery…
1
Protheragen provides development solutions for Machine…
Medical Translation - AI & Medicine
Medical Imaging - AI & Medicine
Medical Therapy and Research System - AI & Medicine
Drug Research and Development Solutions - AI & Medi…
Develop Drug Targets - Protheragen - AI & Medicine
Candidate Drug Discovery - Protheragen - AI & Medic…
Prediction of Drug Crystal Form - Protheragen - AI &…
ADMET Prediction - Protheragen - AI & Medicine
speech recognition https://aimed.protheragen.com/machin…
small molecules https://aimed.protheragen.com/chemical-…
Molecular docking https://aimed.protheragen.com/molecul…
Drug Design https://aimed.protheragen.com/de-novo-drug-…
retrosynthetic route https://aimed.protheragen.com/ai-a…
lead drug https://aimed.protheragen.com/lead-drug-scree…
Structure activity relationship https://aimed.protherag…
Biomarkers https://aimed.protheragen.com/biomarker-disc…
1
ai in medicine https://aimed.protheragen.com/
1
admet prediction https://aimed.protheragen.com/admet-pr…
1
retrosynthetic analysis software https://aimed.prothera…
1
ai and surgery https://aimed.protheragen.com/analysis-o…
1
biomarker synonym https://aimed.protheragen.com/biomark…
1
medical decoding https://aimed.protheragen.com/brain-de…
1
candidate molecule https://aimed.protheragen.com/candid…
1
cough ncp https://aimed.protheragen.com/covid-19-predic…
ai and medicine https://aimed.protheragen.com/
ai in medicine research https://aimed.protheragen.com/
adme t https://aimed.protheragen.com/admet-prediction.h…
admet https://aimed.protheragen.com/admet-prediction.ht…
admet database https://aimed.protheragen.com/admet-pred…
admet phone https://aimed.protheragen.com/admet-predict…
admet prediction https://aimed.protheragen.com/admet-pr…
admet screening https://aimed.protheragen.com/admet-pre…