-
Inspiracje
- Najnowsze
- Najpopularniejsze
- Zaskocz mnie
- Obserwowane
- MAGazyn
- Moda damska
- DIY - zrób to sam
- Kuchnia
- Uroda
- Wnętrza
- Humor
- Ogród
- Ślub
- Architektura
- Biżuteria
- Design
- Dziecko
- Film
- Fotografia
- Gadżety
- Historia
- Hobby
- Inne
- Książka
- Moda męska
- Muzyka
- Nauka i natura
- Plakaty i obrazy
- Podróże i miejsca
- Samochody i motocykle
- Sport i fitness
- Święta i uroczystości
- Tatuaż
- Zwierzęta
- Partnerzy
- KONKURS
-
Zakupy
-
ONA
- Ubrania
- Bielizna
- Bluzki
- Bluzy
- Dresy damskie
- Kombinezony
- Koszule
- Koszulki
- Kurtki
- Płaszcze
- Spodnie i leginsy
- Spodnie jeans
- Spódnice
- Stroje kąpielowe
- Sukienki i tuniki
- Swetry
- Szorty
- Zestawy
- Żakiety i kamizelki
- Buty
- Baleriny
- Botki
- Czółenka
- Espadryle
- Glany
- Japonki
- Kalosze
- Klapki
- Koturny
- Kowbojki
- Kozaki
- Obuwie Domowe
- Półbuty
- Sandały
- Sportowe i lifestyle
- Szpilki
- Tenisówki i Trampki
- Trapery i trekkingi
- Dodatki
- Bransoletki i zawieszki
- Breloki
- Czapki i kapelusze
- Etui
- Kolczyki, nausznice
- Kołnierzyki
- Kosmetyczki
- Naszyjniki, kolie i zawieszki
- Okulary
- Ozdoby do włosów
- Parasole
- Paski
- Pierścionki
- Plecaki
- Portfele
- Rękawiczki
- Skarpety
- Szaliki i chusty
- Torby i torebki
- Walizki
- Zegarki
- Zestawy biżuterii
- Zdrowie i uroda
- Balsamy
- Bazy i podkłady
- Cienie do oczu
- Dezodoranty
- Higiena jamy ustnej
- Korektory
- Kredki
- Kremy i serum
- Lakiery
- Manicure i pedicure
- Maseczki
- Odżywki
- Peeling
- Perfumy i wody perfumowane
- Pielęgnacja po opalaniu
- Płyny żele i mydła
- Pomadki i błyszczyki
- Pudry
- Róże
- Stylizacja włosów
- Szampony
- Toniki i demakijaż
- Tusze
- Wody toaletowe
- Zestawy
-
ON
- Ubrania
- Bielizna
- Bluzy
- Kąpielówki
- Koszule
- Kurtki i płaszcze
- Marynarki kamizeki
- Spodenki i szorty
- Spodnie
- Swetry
- T-shirt długi rękaw
- T-shirt i Polo
- KULTURA
- GADŻETY
- DZIECKO
-
DOM I WNĘTRZE
- Wnętrza
- Akcesoria dom
- Ceramika i szkło
- Do baru
- Do biura
- Do kuchni
- Kubki kufle i kieliszki
- Lustra
- Nakrycia stołu
- Oświetlenie
- Plakaty i tablice
- Pościele, poduszki i nakrycia
- Przechowywanie
- Tekstylia
- Zegary i budziki
-
Marki
- MARKI
- Adidas
- Adriatica
- Alter Core
- Armani
- Asics
- Atlantic
- Azzaro
- Barbie
- Bburago
- Be-U
- Benetton
- Bering
- Black Plum
- Burberry
- Bvlgari
- Cacharel
- Calvin Klein
- Canon
- Carolina Herrera
- Carrera
- Casio
- Celestron
- Chanel
- Chloe
- Clinique
- Cobi
- Coloud
- Converse
- David Beckham
- Davidoff
- Delbana
- Diesel
- Dior
- DISNEY
- DKNY
- Dolce & Gabbana
- DOXA
- Dr. Martens
- Dunhill
- Ecco
- Elizabeth Arden
- Esprit
- EVC DSGN
- Festina
- Fila
- Fisher Price
- Frederique Constant
- Givenchy
- Gucci
- Guerlain
- Guess
- Hasbro
- Helena Rubinstein
- Hermes
- Hi-Tec
- Hugo Boss
- Hunter
- Iceberg
- Ingersoll
- Issey Miyake
- Jean Paul Gaultier
- Jennifer Lopez
- Jil Sander
- Jimmy Choo
- Joop!
- Juicy Couture
- Kappa
- Kenzo
- Komono
- Lacoste
- Lalique
- Lancome
- Lanvin
- Lee
- Lego
- Lorus
- Marc Jacobs
- Marshall
- Masaki Matsushima
- Matchbox
- Mattel
- Max Factor
- Max&Co
- Mc Arthur
- Mizuno
- Mont Blanc
- Moschino
- Mr. Gugu & Miss Go
- My Little Pony
- New Balance
- Nike
- Nikon
- Nina Ricci
- Ninety Eight Clothing
- Nivea
- Nixon
- Nooka
- Obaku
- Onitsuka Tiger
- Paco Rabanne
- Pentax
- Pewex
- Pierre Cardin
- Playboy
- Prada
- Puma
- Ralph Lauren
- Reebok
- Regatta
- Revlon
- Rexona
- Rider
- Salomon
- Salvatore Ferragamo
- Shelyak
- Shiseido
- Skagen
- Swarovski
- Takahashi
- Thierry Mugler
- Timberland
- Timex
- Tommy Hilfiger
- UGC
- Urbanears
- Valentino
- Vans
- Versace
- Viktor & Rolf
- Vixen
- Welly
- William Optics
- Wrangler
- Yimaida
- Yukon
- Yves Saint Laurent
-
ONA
-
Szukaj
- Losuj
Inne
protheragen
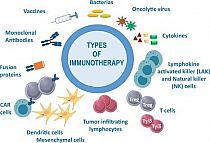
The Common Technical Document (CTD) is a set of specifications for a dossier to register medicines. After the United States, European Union and Japan, the CTD has been adopted by several other countries including Canada, Australia and Switzerland. The Paper CTD is destined to be replaced by its electronic counterpart, the eCTD. Common Technical Document Translation; CTD Translation
https://medtrans.protheragen.com/common-technical-document-translation.html
https://medtrans.protheragen.com/common-technical-document-translation.html
MA==
Podobne inspiracje
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides development solu…
Protheragen AI & Medicine provides comprehensive so…
Protheragen provides effective AI solutions for Image D…
Protheragen provides effective AI solutions for Surgery…
1
Protheragen provides development solutions for Machine…
Medical Translation - AI & Medicine
Medical Imaging - AI & Medicine
Medical Therapy and Research System - AI & Medicine
Drug Research and Development Solutions - AI & Medi…
Develop Drug Targets - Protheragen - AI & Medicine
Candidate Drug Discovery - Protheragen - AI & Medic…
Prediction of Drug Crystal Form - Protheragen - AI &…
ADMET Prediction - Protheragen - AI & Medicine
speech recognition https://aimed.protheragen.com/machin…
small molecules https://aimed.protheragen.com/chemical-…
Molecular docking https://aimed.protheragen.com/molecul…
Drug Design https://aimed.protheragen.com/de-novo-drug-…
retrosynthetic route https://aimed.protheragen.com/ai-a…
lead drug https://aimed.protheragen.com/lead-drug-scree…
Structure activity relationship https://aimed.protherag…
Biomarkers https://aimed.protheragen.com/biomarker-disc…
1
ai in medicine https://aimed.protheragen.com/
1
admet prediction https://aimed.protheragen.com/admet-pr…
1
retrosynthetic analysis software https://aimed.prothera…
1
ai and surgery https://aimed.protheragen.com/analysis-o…
1
biomarker synonym https://aimed.protheragen.com/biomark…
1
medical decoding https://aimed.protheragen.com/brain-de…
1
candidate molecule https://aimed.protheragen.com/candid…
1
cough ncp https://aimed.protheragen.com/covid-19-predic…
ai and medicine https://aimed.protheragen.com/
ai in medicine research https://aimed.protheragen.com/
adme t https://aimed.protheragen.com/admet-prediction.h…
admet https://aimed.protheragen.com/admet-prediction.ht…
admet database https://aimed.protheragen.com/admet-pred…
admet phone https://aimed.protheragen.com/admet-predict…
admet prediction https://aimed.protheragen.com/admet-pr…
admet screening https://aimed.protheragen.com/admet-pre…